Life and Ramdom Events
Original Post: 25 May 2012
Posted Here: 4 December 2017
 Many of the events which occur in the universe are random. There is no way to predict what will happen. No one knows which particular atom of carbon-14 in a piece of ancient charcoal will decay and release radiation. No one knows which of a life insurance company’s clients will die this year. Statistical mathematics can be used to provide the probability of how many events will occur, but not which events.
Many of the events which occur in the universe are random. There is no way to predict what will happen. No one knows which particular atom of carbon-14 in a piece of ancient charcoal will decay and release radiation. No one knows which of a life insurance company’s clients will die this year. Statistical mathematics can be used to provide the probability of how many events will occur, but not which events.
I can recall hearing mathematical arguments being presented to show that enzymes, for example, could not have been created by random events, they must have been designed. An enzyme (a protein) is made up like a string of beads of twenty components called amino acids. If we have an enzyme that is 100 amino acids long, then there are 1.27 x 10^130 (127 followed by 128 zeros) different proteins that could be formed “randomly” from those twenty types of amino acids. If, according to Wikipedia, the universe contains only about 10^50 atoms (1 followed by 50 zeros), then we don’t have enough atoms in the universe to form all of those enzymes. So there is no way that evolution could have selected one of those possible sequences to use in a living thing. This enzyme, and all others, must have been created by a designer.
 But this conclusion is based on some fallacies. The first is that a specific enzyme or protein is a unique sequence of amino acids. An enzyme is defined by its function, not its sequence. Hemoglobin, the protein which carries oxygen in your blood, is different in different species. It can even be different in different people. Yes, all hemoglobins are similar. But similar is not identical. We don’t know why this particular family of proteins acts as hemoglobin in different species. But that does not mean that no other family would function just as well. Ignorance is not evidence. Hemoglobins are not, in fact, the only proteins whose function is to carry oxygen in the blood. Hemocyanins have this function in some invertebrates, such as horseshoe crabs.
But this conclusion is based on some fallacies. The first is that a specific enzyme or protein is a unique sequence of amino acids. An enzyme is defined by its function, not its sequence. Hemoglobin, the protein which carries oxygen in your blood, is different in different species. It can even be different in different people. Yes, all hemoglobins are similar. But similar is not identical. We don’t know why this particular family of proteins acts as hemoglobin in different species. But that does not mean that no other family would function just as well. Ignorance is not evidence. Hemoglobins are not, in fact, the only proteins whose function is to carry oxygen in the blood. Hemocyanins have this function in some invertebrates, such as horseshoe crabs.
The second fallacy is that proteins must have been the result of random events. Chemistry is not governed by random events. What atom collides with what other atom when a reaction occurs may be a random event. But what happens during that collision is far from random. One of the first things that High School Chemistry students learn is that chemical reactions are not random. Put hydrogen and oxygen together and add a spark or flame. What happens? Boom! Plaster falls off the ceiling. What do we get? Water: H2O. Not HO4 not H3O2, not HO2, not… Not anything else. Just water. Another compound of hydrogen and oxygen does exist—hydrogen peroxide. H2O2. But just that one. The collisions between hydrogens and oxygens might be random, but what happens next is not random. The number of atoms that hydrogen, oxygen, carbon or any other element reacts with is not random.
One of the first things that students in Organic Chemistry learn in college is that organic compounds (carbon compounds—amino acids and proteins are organic compounds) do not react randomly. Benzene is an organic compound made of six carbon atoms arranged in a ring (at the left in the reaction below—carbons are at the corners, just not shown). If a bromine molecule reacts with it to add just one atom, we get the compound, bromobenzene, shown in the reaction below.
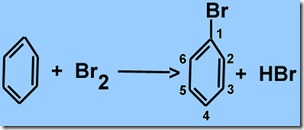
The bromine added “randomly” to any of the six identical carbons. Suppose we want to add a second bromine. If this added randomly, then we would expect to find similar amounts of three different products—where the second bromine was added to positions numbered 1, 2 or 3 (because the molecule can be flipped left to right, products with a bromine at positions 2 or 6 are really the same; likewise for positions 3 and 5.) What we actually find are two main products: Molecules with a second bromine at position 2 (or 6) and 4. Very little bromine attaches to carbon 3 (or 5). That is hardly to be considered “random.”
This is not an unusual reaction. Both inorganic and organic chemical reactions are definitely nonrandom. So, while we may not yet know why a specific amino acid sequence is to be found in a particular protein, we can feel sure that it didn’t require some designer to pick the right one out of a zillion random possibilities. Abiologically formed proteins won’t have random sequences (even if they look random) and a variety of families of these proteins probably exist that could have a desired biological function.
Keep reading/keep writing – Jack